|
5th ASME/JSME Joint Thermal Engineering Conference March 15-19, 1999, San Diego, California |
AJTE99/6134
Determination of the Spread Rate for Downward Flame Spread Over Thick Fuels
|
Matthew D. King Subrata Bhattacharjee San Diego State University Department of Mechanical Engineering San Diego, California, 92182 USA Ph: (619)594-6080, Fax: (619)594-3599 mking@rohan.sdsu.edu, subrata@rohan.sdsu.edu |
Keywords
Flame Spread, Natural Convection, Numerical
PMMA, Spread Rate Formula
Abstract
An evaluation of various formulae and correlations for downward flame spread in over solid fuels in the thermal regime suggests that an easy-to-apply working formula for the prediction of the spread rate is still missing. Here, a recently developed simplified approach, capable of predicting the spread rate and flame structure for forced opposed flow, is extended to the downward configuration by proposing an equivalent bouyant convection velocity and a new characteristic temperature equal to the fuel vaporization temperature. The predictions are compared with numerical and available experimental results over a wide range of fuel thickness, pressure, gravity levels, and oxidizer concentration.
Introduction
Considerable work has been done over the last three decades on the fundamental opposed-flow flame spread problem. Because the induced flow generated by buoyancy is, in general, vertically upward near the vicinity of the flame leading edge, downward flame spread can be treated as an extension of the opposed-flow regime.
In this study we will build upon a recently proposed (Bhattacharjee et al., 1996) opposed flow flame spread formula to extend it to the downward configuration. The resulting formula will be tested against a comprehensive numerical model, various experimental works and the existing formulae.
Nomenclature
Symbols (Units)
![]() Constant
Constant
![]() Specific heat, (
Specific heat, (![]() )
)
F, F' Vaporization constants
![]() Reference length, (m)
Reference length, (m)
![]() Gravitational acceleration, (m/s2)
Gravitational acceleration, (m/s2)
![]() Temperature of the gas, (
Temperature of the gas, (![]() )
)
![]() Vaporization temperature, (
Vaporization temperature, (![]() )
)
![]() Velocity, (
Velocity, (![]() )
)
Greek Symbols
![]() Thermal diffusivity, (
Thermal diffusivity, (![]() )
)
![]() Non-dimensional parameter
Non-dimensional parameter
![]() Hydrodynamic coefficient
Hydrodynamic coefficient
![]() Thermal conductivity, (
Thermal conductivity, (![]() )
)
![]() Density, (
Density, (![]() )
)
![]() Fuel half-thickness, (m)
Fuel half-thickness, (m)
Subscripts
BC Buoyant Convection
c characteristic
e Earth
eqv Equivalent
EST Extended Simplified Theory
f Flame
g Gas
hd Hang distance
¥
Ambient conditionss Solid
Thick Thermally thick fuel
x Direction parallel to flame
Thin Thermal thin fuel
Numerical Analysis
With regard to the difficulty in conducting combustion experiments with adequate variations of significant parameters, a well validated numerical model, including finite-rate kinetics, single step chemistry, gas phase kinetics and radiation, including gas-to-surface radiation feedback, will be employed in this work. The numerical model is capable of reproducing flame spread rates and the field variables for the Emmons problem, de Ris solution for thin and thick fuels, and several other benchmarks giving us confidence in it's accuracy. (Bhattacharjee and Altenkirch, 1990, Bhattacharjee et al., 1996, King, 1996, Thomas, 1995)
SIMPLIFIED APPROACH
Bhattacharjee et al. (1996) proposed a flame spread formula that retained the form of the de Ris formula for forced opposed-flow flame spread in the thermal regime.
The formula for thick and thin fuels were proposed as:
![]() (1)
(1)
and 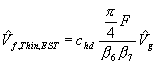 (2)
(2)
where, ![]() is the temperature term used by de Ris,
is the temperature term used by de Ris, ![]() is a modified temperature term that accounts for flame lift-off over thick fuels, and
is a modified temperature term that accounts for flame lift-off over thick fuels, and ![]() is an equivalent opposed flow velocity (correlated to the boundary layer development ahead of the flame) that replaces the average opposing velocity
is an equivalent opposed flow velocity (correlated to the boundary layer development ahead of the flame) that replaces the average opposing velocity ![]() for thick fuels and the
for thick fuels and the ![]() ’s are known non-dimensional parameters of the problem.
’s are known non-dimensional parameters of the problem.
![]() For thin fuels, the de Ris-Delichatsios formula is modified with the hang-distance correction factor addressing the overhang of the flame leading edge ahead of the pyrolysis front (Bhattacharjee, 93).
For thin fuels, the de Ris-Delichatsios formula is modified with the hang-distance correction factor addressing the overhang of the flame leading edge ahead of the pyrolysis front (Bhattacharjee, 93).
The buoyant extension of this approach, as recommended by de Ris (1969), involves the replacement of the forced flow equivalent velocity, ![]() , with its buoyant counterpart, determined here by scaling.
, with its buoyant counterpart, determined here by scaling.
(3)
where, ![]() is an appropriate characteristic gas temperature, and cBC is an undetermined constant.
is an appropriate characteristic gas temperature, and cBC is an undetermined constant.
The adiabatic flame temperature Tf has often been used as the characteristic temperature, but there has been some debate as to the most appropriate choice. Wichman (Wichman, 1983), (Wichman and Saito, 1983) made use of flame temperatures and combinations of vaporization and ambient temperatures in various theories.
In this work we propose a hypothesis that it is the fuel vaporization temperature, ![]() , that is the appropriate characteristic temperature. The spread rate is controlled by the forward heat transfer at the leading edge of the flame, and computational (King, 1996) and experimental (Bundy, 1995) temperature fields indicate that in the vicinity of the leading edge temperature is moderated by the fuel vaporization and remains in the order of
, that is the appropriate characteristic temperature. The spread rate is controlled by the forward heat transfer at the leading edge of the flame, and computational (King, 1996) and experimental (Bundy, 1995) temperature fields indicate that in the vicinity of the leading edge temperature is moderated by the fuel vaporization and remains in the order of ![]() .
.
The characteristic temperature, ![]() , was therefore taken to be the constant vaporization temperature which was shown (Bhattacharjee et. al, 1994) to vary by less than 5% for wide variation of oxygen level. For a
, was therefore taken to be the constant vaporization temperature which was shown (Bhattacharjee et. al, 1994) to vary by less than 5% for wide variation of oxygen level. For a ![]() , the coefficient of buoyant convection was determined by a best fit of the proposed approach to experiments resulting in cBC =0.575. The details behind the evaluation of this constant can be found elsewhere (King, 1996).
, the coefficient of buoyant convection was determined by a best fit of the proposed approach to experiments resulting in cBC =0.575. The details behind the evaluation of this constant can be found elsewhere (King, 1996).
A JAVA applet that calculates the spread rate for the downward configuration using Eqs. (1)-(3) has been posted in the following web site: http://eng.sdsu.edu/.
Results and Discussion
Comparison with Experimental and Numerical Results
Downward flame spread rates for thick PMMA at various oxygen concentrations in atmospheric pressure is shown on Fig. 1. The proposed approach (solid line) is overlaid on the experimental data of Fernandez-Pello et al. (1981) (solid squares), the finite-rate chemical kinetic numerical results (open squares), and the infinite-rate chemical kinetic numerical results (open circles).
The finite-rate numerical results and experiments match very well throughout the entire range of available data.
The finite-rate computations also show excellent agreement with the infinite-rate computations down to about the 30% O2 level, establishing it as the critical level that delineates the thermal regime from the kinetic regime of downward spread for thick PMMA at 1 atmosphere. This is consistent with the observations made previously by Wichman et al. (1982).
The infinite-rate model does not include radiation, yet in the thermal regime it agrees with the finite-rate kinetics model which does include radiation, indicating that radiation is unimportant in downward spread in the thermal regime.
The prediction from proposed formula (Eqs. 1-3), the solid line in Fig. 1, can be seen to agree well with the infinite-rate computations at all oxygen concentrations, establishing it as a reasonably accurate formula for the thermal regime where chemistry can be considered fast relative to the residence time.
The variation of spread rate with fuel half-thickness, ![]() , for selected O2 concentrations is plotted in Fig. 2. As before, the numerical results (empty symbols), experimental results (filled symbols), and the simplified approach predictions (solid lines) are compared. In the thermal regime the prediction agrees quite well with experimental results, available only for the thick limit. It also agrees quite well with the numerical spread rate for both the thin and thick limit and captures the appropriate transition thickness between the two limits.
, for selected O2 concentrations is plotted in Fig. 2. As before, the numerical results (empty symbols), experimental results (filled symbols), and the simplified approach predictions (solid lines) are compared. In the thermal regime the prediction agrees quite well with experimental results, available only for the thick limit. It also agrees quite well with the numerical spread rate for both the thin and thick limit and captures the appropriate transition thickness between the two limits.
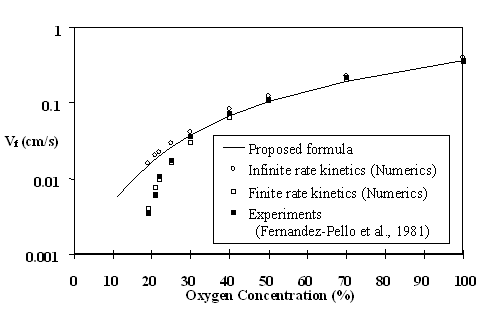
Figure 1: Spread rate vs. O2 level for thick PMMA at p=1 atm. and ![]() . Finite-rate chemistry effect is evident below 30% O2.
. Finite-rate chemistry effect is evident below 30% O2.
Finite-Rate Kinetics
The finite-rate computations and the experimental spread rates diverge from the infinite-rate computations or predicted spread rate from about 30% oxygen level. One way of quantifying the finite-rate effects, which increases as the O2 level decreases, is to define an equivalent O2 level at which the proposed formula predicts the same spread rate as the experiment or the finite-rate computations. The equivalent O2 level corresponding to the ambient oxygen level can be obtained from Fig. 1 as 11%. It remains to be seen if such a description of finite-rate effects can be extended to other fuel thickness.
The data obtained for 21%, shown in Fig. 2, comes from three different experiments. The experimental results and computations for a given thickness can be seen to be significantly less than the prediction at 21%, as expected. Note that the transition thickness significantly increases as a result of the finite-rate kinetics. The thermal-regime prediction at 11% O2 (Fig. 2) can be seen to capture both of these effects quite accurately. Further investigation is needed before this encouraging indication – that a thermal-regime formula at a reduced oxygen level can capture the finite-rate effects - can be established firmly.
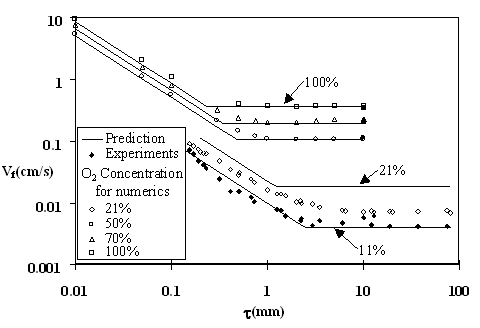
Figure2: Spread rate vs. fuel thickness (PMMA at p=1 atm. and ![]() ) at different O2 levels. The prediction is compared with computations and experiments (Fernandez-Pello and Hirano, 1983, Fernandez-Pello et. al., 1981, Fernandez-Pello and Williams, 1975, Sibulkin et. al., 1976).
) at different O2 levels. The prediction is compared with computations and experiments (Fernandez-Pello and Hirano, 1983, Fernandez-Pello et. al., 1981, Fernandez-Pello and Williams, 1975, Sibulkin et. al., 1976).
Comparison with other formulae
The proposed formula is compared with other available formulae: Wichman (1983, dot-dashed line), de Ris (1969, large dashed line), Fernandez-Pello and Williams (1975, small dashed line), and experimental results (Fernandez-Pello et al., 1981, solid squares), in Fig. 3. Once again we plot the spread rate, ![]() , against O2 concentration.
, against O2 concentration.
Constant coefficients were introduced into the other formulae to match the experimental and numerical results at 100% O2. Without this constant there would be large discrepancies between the experimental data and theories.
The best of the Wichman formulae (Wichman and Saito, 1983) for the thermal regime, demonstrates an inappropriate dependence on oxygen concentration. Taking into account flame temperatures, as per their model, their bouyant theory over predicts the spread rate at 30% O2 by 55%. The proposed equation for ![]() differs from the Wichman equation by choice of the characteristic temperature, and the flame spread equation in which it is used also differs in its temperature dependence.
differs from the Wichman equation by choice of the characteristic temperature, and the flame spread equation in which it is used also differs in its temperature dependence.
The de Ris model offers the best fit of the data down to 21% oxygen, but also only applies to the thermal regime. It generates predictions that are 27% low at 30% O2 concentration.
Similarly, Fernandez-Pello and Williams (1975) correlation generates predictions that are 36% low at 30% O2 concentration.
The proposed approach demonstrates the best fit with regard to oxygen dependence in the thermal regime, over predicting the spread rate at 30% O2 by only 8%.
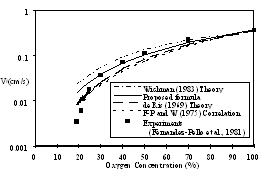
Figure 3: Proposed formula compared with selected theories and experiments for the same conditions as in Fig. 1.
Effect of gravity level
Figure 4, demonstrates the ability of this approach to predict the flame spread rates at selected oxygen concentrations over a range of gravity levels. The spread rate data is plotted against ![]() , which can be compared to the Vg sweeps found in the forced flow literature. In the thermal regime,
, which can be compared to the Vg sweeps found in the forced flow literature. In the thermal regime, ![]() is expected to increase linearly with opposing flow velocity (which scales with
is expected to increase linearly with opposing flow velocity (which scales with ![]() ) and fall off when the blow-off extinction process kicks in at high
) and fall off when the blow-off extinction process kicks in at high ![]() . Numerical results (open symbols), simplified approach prediction (solid lines), and the Fernandez-Pello and Williams (1975) correlation (dashed line) are presented here. Figure 4 shows once again that combustion at the higher oxygen concentrations is well into the thermal regime for low and moderate gravity levels. At very high gravity levels, as the induced opposing flow reaches a critical value, the onset of the blow-off regime is noticeable. This is much more pronounced at 21% where the finite-rate effect is always present.
. Numerical results (open symbols), simplified approach prediction (solid lines), and the Fernandez-Pello and Williams (1975) correlation (dashed line) are presented here. Figure 4 shows once again that combustion at the higher oxygen concentrations is well into the thermal regime for low and moderate gravity levels. At very high gravity levels, as the induced opposing flow reaches a critical value, the onset of the blow-off regime is noticeable. This is much more pronounced at 21% where the finite-rate effect is always present.
It is clear that the Fernandez-Pello and Williams (1975) correlation is matched to experiments for 21% oxygen at moderate gravity levels, but since this is in the blow-off regime, the gravity dependence from their formula (dotted line) cannot apply to the thermal regime.
The gravitational dependence of this simplified approach is essentially the same as in the other formulae, and has been fairly well established for ideal vaporizing solids such as PMMA.
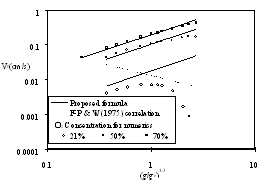
Figure 4: Effect of gravity level for thick PMMA at three oxygen levels at 1 atm. At high g-level, the blow-off regime is reached and the finite-rate computations deviates from the thermal-regime predictions.
Effect of Pressure
The effect of ambient pressure on downward spread rate in the thermal regime (O2 level 40%) is shown in Fig. 5 where we compare the simplified approach prediction (solid line), to the numerical results (empty squares), and the experiments (filled squares) of Altenkirch et al. (1983).
While the simplified approach produces the same trend as the experiments and computations, the discrepancy appears to increase with pressure. This is possibly due to inclusion of radiation in the numerical model which the simplified theory neglects. At lower pressure the radiation effects seem to be insignificant.
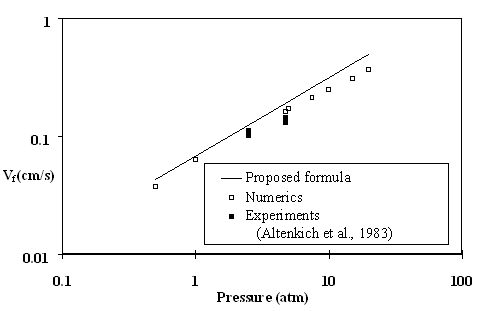
Figure 5: Effect of ambient pressure for thick PMMA at 40% oxygen level at ![]() . At high pressure, radiative effects cause the computational spread rates to fall-off below the thermal-regime prediction.
. At high pressure, radiative effects cause the computational spread rates to fall-off below the thermal-regime prediction.
Conclusion
In spite of the existing theories an accurate, easy-to-use, closed-form formula with no undetermined constants for the prediction of downward spread rate over solid fuels is still lacking. The simplified formula presented here extends de Ris formula into the downward configuration through the use of an equivalent velocity to represent buoyancy-induced flow and a characteristic temperature, equal to the fuel vaporization temperature, for evaluation of all properties including the equivalent velocity. The proposed formula has been compared with available experimental results and extensive numerical simulation varying fuel thickness, ambient oxygen level, ambient pressure and the gravity level with excellent agreement throughout the thermal regime.
For downward spread over PMMA placed in a O2-N2 mixture at atmospheric pressure, the proposed formula matches experiments and computations using infinite-rate and finite-rate kinetics for oxygen level above 30%. The finite-rate effect at the ambient oxygen level has been shown to be reproduced by the proposed formula with a reduced oxygen level of 11%.
Radiative effects do not seem to influence the spread rate except at very high ambient pressures. The blow-off kinetic limit has been shown to appear only at very high g-levels.
A Java applet that evaluates downward spread rate using the proposed formula is posted on the web page: http://eng.sdsu.edu/ for the convenience of the research community.
References
Altenkirch, R.A., Eichorn, R., and Rizvi, A.R., 1983, "Correlating Downward Flame Spread Rates for Thick Fuel Beds." Combustion, Science and Technology, 32, pp. 49-66.
Bhattacharjee, S., "A Comparison of Numerical and Analytical Solution of the Creeping Flame Spread Over Thermally Thin Material", Combustion and Flame, Vol. 93, pp. 434-444, (1993).
Bhattacharjee, S., Bhaskaran, K.K. and Altenkirch, R. A., 1994, "Effects of Pyrolysis Kinetics on Opposed-Flow Flame Spread Modeling", Combustion Science and Technology, Vol. 100, pp. 163-182
Bhattacharjee, S., and Altenkirch, R.A., 1990, "Radiatively Controlled Flame Spread", Twenty-Third Symposium (International) on Combustion, The Combustion Institute, Pittsburgh, pp. 1627-1633.
Bhattacharjee, S., West, J., and Altenkirch, R. A., 1996, "Determination of the Spread Rate in Opposed-Flow Flame Spread Over Thick Solid Fuels in the Thermal Regime.", Twenty-Sixth Symposium (International) on Combustion, The Combustion Institute, Pittsburgh, PA, pp. 1477-1485.
Bundy, M., 1995, "Flame Tracker - Development and testing of a New Experimental Device for investigating Downward Spreading Flames." Masters Thesis, San Diego State University.
de Ris, J., 1969, "Spread of a Laminar Diffusion Flame." Twelfth Symposium (International) on Combustion, The Combustion Institute, pp. 241-252.
Fernandez-Pello, A.C., Ray, S.R., and Glassman, I., 1981, "Flame Spread in an Opposed Forced Flow: The Effect of Ambient Oxygen Concentration." Eighteenth Symposium (International) on Combustion, The Combustion Institute, Pittsburgh, PA, pp. 579-581.
Fernandez-Pello, A.C., and Williams, F.A., 1977, "A Theory of Laminar Flame Spread Over Flat Surfaces of Solid Combustibles." Combustion and Flame, 28, pp. 251-277.
Fernandez-Pello, A.C., and Williams, F.A., 1975, "Laminar Flame Spread over PMMA surfaces." Fifteenth Symposium (International) on Combustion, The Combustion Institute, Pittsburgh, PA, pp. 217-231.
King, M., 1996, "A Simplified Theory for Downward Flame Spread" Masters Thesis, San Diego State University.
Thomas, P., 1995, "A Numerical Investigation of Burning Rates of Spreading and Non-Spreading Flames in Opposed Flow Configuration" Masters Thesis, San Diego State University.
Wichman, I. S., 1983, "Flame Spread in an Opposed Flow with a Linear Velocity Gradient." Combustion and Flame, 50, pp. 287-304.
Wichman, I. S., and Saito, K., 1983, "An Experimental Study of the Effects of Gravity on Flame Spread in High Oxygen Concentration Environments." Combustion and Flame, 52, pp. 291-297.
Wichman, I. S., Williams, F. A., and Glassman, I., 1982, "Theoretical Aspects of Flame Spread in an Opposed Flow Over Flat Surfaces of Solid Fuels." nineteenth Symposium (International) on Combustion, The Combustion Institute, Pittsburgh, PA, pp. 835-845.